Attention
This website is best viewed in portrait mode.
-
industries
- healthcare and life sciences
- medical device design and engineering
-
Medical Device Engineering
Medical Device Engineering
Research | Design | Deploy

Medical Device Engineering
Research | Design | Deploy
Building Next-Gen Medical Devices

In today's highly competitive medical device landscape, OEMs need to launch superior and differentiated products to stay relevant. As the demand for innovation and patient-centric solutions surge, the ability to create cutting-edge medical devices becomes not just a competitive advantage, but a necessity.
Further, technologies like cloud computing, AI, machine learning, miniaturization, and analytics are making medical devices smarter and more connected. These advancements in medical device engineering are leading to improved patient experiences, streamlined healthcare workflows, and enhanced diagnostic accuracy.
Our medical device engineering services cover every stage of product development, from concept validation to design and regulatory compliance. We have expertise in medical device hardware, firmware, mechanical engineering, verification & validation, test automation, and product lifecycle management. Additionally, we leverage our digital COEs, partnership ecosystem, and solution accelerators to optimize R&D costs, thereby accelerating the time-to-market.




Patti White
"In our strategic mission to develop and launch a Lab-in-a-Box product, we are delighted to have the right partner in Tata Elxsi who understands the challenges of markets and possesses comprehensive capabilities from concept development to engineering and launch of medical products that meet regulatory standards."
Chief Executive Officer, Hemex Health

Here’s How We Can Help Medical Device OEMs

Launch Superior and Differentiated Products in the Market
- End-to-end product development covering all aspects, from inception to commercialization.
- Integrate digital technologies (IoMT, AI,AR/ VR/MR) for next-gen devices, enabling diverse use cases and fostering innovation.
Improve Device Reliability, Performance and Safety
- Rigorous testing aligned with ISO 17025 and ISO 14971 standards to guarantee top-notch device quality.
- Cybersecurity expertise, backed by standards such as UL2900 and IEC 62443 to safeguard devices against evolving threats.
Sustain High Revenue-Generating Products in the Market
- Post-market engineering, encompassing obsolescence and value engineering, extending the product lifecycle.
- Ensure proactive compliance with evolving regulatory standards, such as EU MDR/IVDR, FDA 21 CFR Part 820, ISO 13485, etc. to safeguard your market presence.
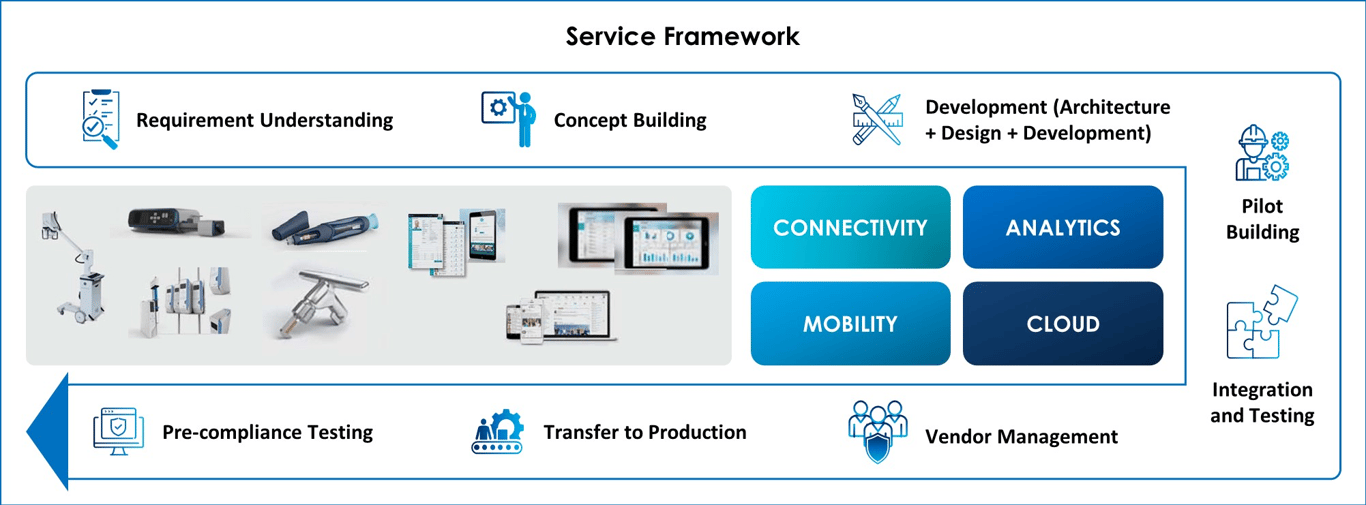
Medical Device Engineering Services Framework
Why Tata Elxsi?
- End-to-End Product Development with faster time-to-market using lean project management methodologies
- Flexible engagement models conducive to innovation and research-led product engineering
- Strategic partnerships with a large group of medical device components suppliers and premier institutes for research and innovation
- Exhaustive QMS and product realization process
- ISO 13485:2016 design facilities
In Focus
August 05, 2024
Press Releases
Skanray Partners with Tata Elxsi to Advance Surgical Imaging and Healthcare Innovation

March 28, 2024
Press Releases
Tata Elxsi and Dräger Establish an Innovative Partnership to Drive Critical Care Innovation in India

December 06, 2021
Press Releases
Tata Elxsi recognized as a ‘Leader’ and specialized ER&D Service Provider across multiple industries in Zinnov Zones 2021 annual ratings
Subscribe
To subscribe to the latest updates & newsletter