Attention
This website is best viewed in portrait mode.
-
industries
- healthcare and life sciences
- medical device design and engineering
-
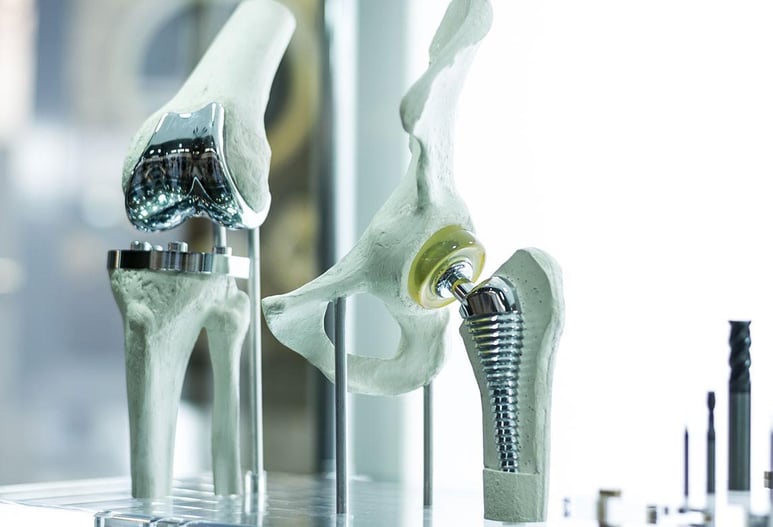
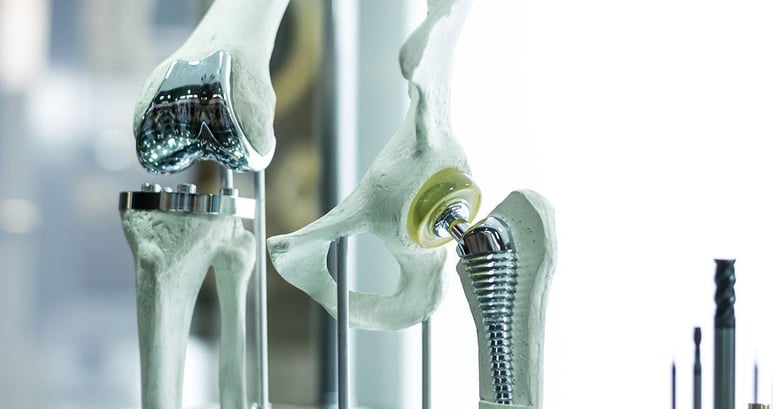
Medical Device Mechanical Design and Engineering
Medical Device Mechanical Design and Engineering
CAE | CAD | Manufacturing Process Simulation
Medical Device Mechanical Design and Engineering
CAE | CAD | Manufacturing Process Simulation
Increasing Speed-to-Market with Reduced Development Risks

The global medical devices market size is poised to grow from USD 63.4 billion in 2022 to USD 134.56 billion by 2030. (Source: Fortune Business Insights)
In this competitive medical device market, healthcare OEMs grapple with numerous challenges such as navigating stringent regulations, ensuring device quality, balancing innovation and costs, fostering collaboration, and keeping up with the latest technological advancements. This prompts them to seek expertise from MedTech solution providers for cutting-edge design and engineering.
Tata Elxsi is your perfect partner in this journey, with over two decades of comprehensive medical device mechanical design and engineering expertise across New Product Development, automated manufacturing and product innovation and improvement.


Here’s How We Can Help You

Faster Speed-to-Market
- Model-based analysis to foresee roadblocks during the development phase
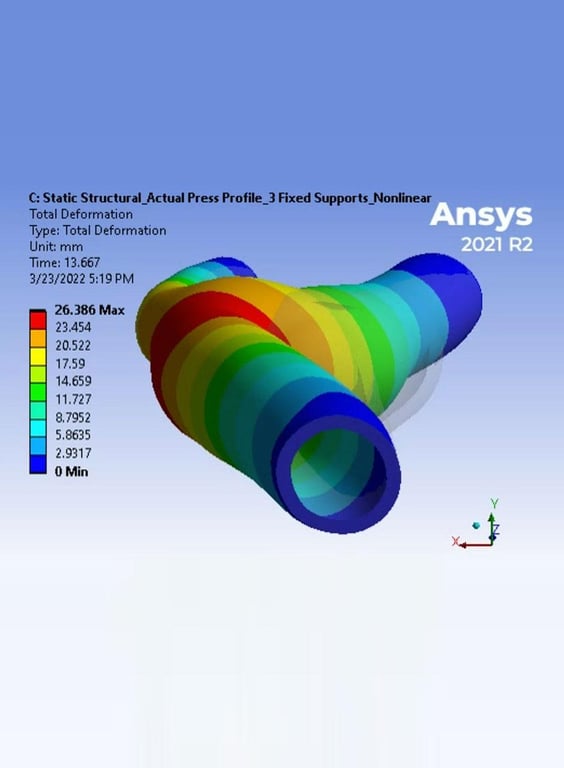
- Digital Simulation (CAE), Structural, Mold Flow Analysis, Development Support, Verification and Validation
- Manufacturing Feasibility Simulations
Expertise in providing solutions for Advanced Robotics and Automation of Healthcare Processes in:
- Robust production assembly
- Rapid prototyping with Additive Manufacturing
- CAD and Industrial Design support
Regulations and Compliance - ISO, FDA, EU MDR
- Design Modifications and Development Support
- Process Standardisation
- Verification and Validation
Service Framework

Why Tata Elxsi?
- ISO 13485:2016 certified design facilities
- Expertise in additive manufacturing design approach of components
- Over two decades of experience in Product Engineering, Testing and Analysis
Subscribe
To subscribe to the latest updates & newsletter