Attention
This website is best viewed in portrait mode.
-
industries
- healthcare and life sciences
- medical device design and engineering
-
Human Factors & Usability Engineering
Human Factors & Usability Engineering for Medical Devices
Research & Design | Validate | Deploy

Human Factors & Usability Engineering for Medical Devices
Research & Design | Validate | Deploy
Designing Safe, Efficient, and Easy-to-Use Medical Devices
The rise of Telehealth, Remote Monitoring and Digital Health has led to the democratization of healthcare diagnosis. This is causing a paradigm shift in medical device usability testing and product design. With the easy availability of medical devices to diverse user groups (patients/caregivers), it is imperative to optimize usability and provide safe care without compromising on quality.
The increasing emphasis on compliance of medical devices with FDA, EU, and MDR standards is further accentuating the importance of Human Factors and Usability Engineering in the Healthcare and Pharma space.
Tata Elxsi has expertise in Human Factors and Usability Engineering. We assist global medical device manufacturers and pharmaceutical companies in designing and developing products that optimize performance. Tata Elxsi's usability engineering solutions for medical devices ensure that products are not only efficient but also user-friendly.


Here’s How We Can Help You

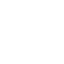
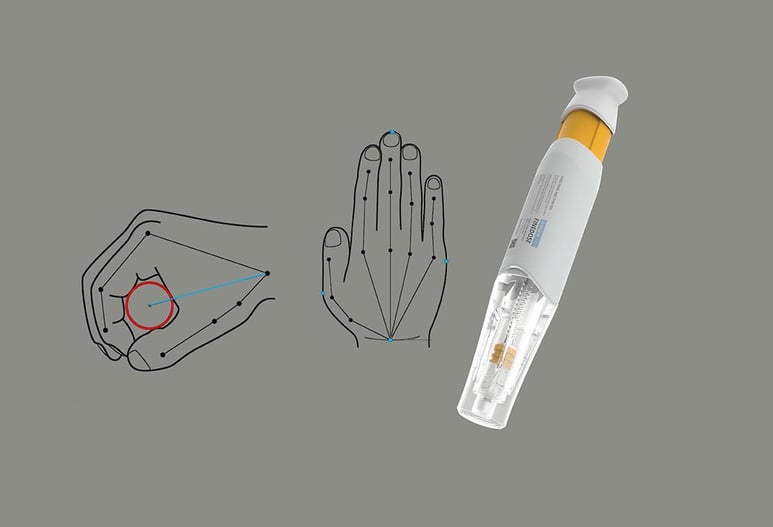
Simplifying design methodology through usability engineering medical devices
- Provide formative and summative evaluations and design Research based on the right user group to improve overall product design.
In-depth analysis of learning preferences for developing device training materials
- To enhance the learning experience of each user group, we work across - usability testing, clinical trials, usability validation, analysis of user journey and user experience design.
Enabling medical manufacturers to adhere to stringent Regulatory Compliance
- Reporting and IRB submissions, Risk management, Design with compliance to Human Factors Engineering and Usability
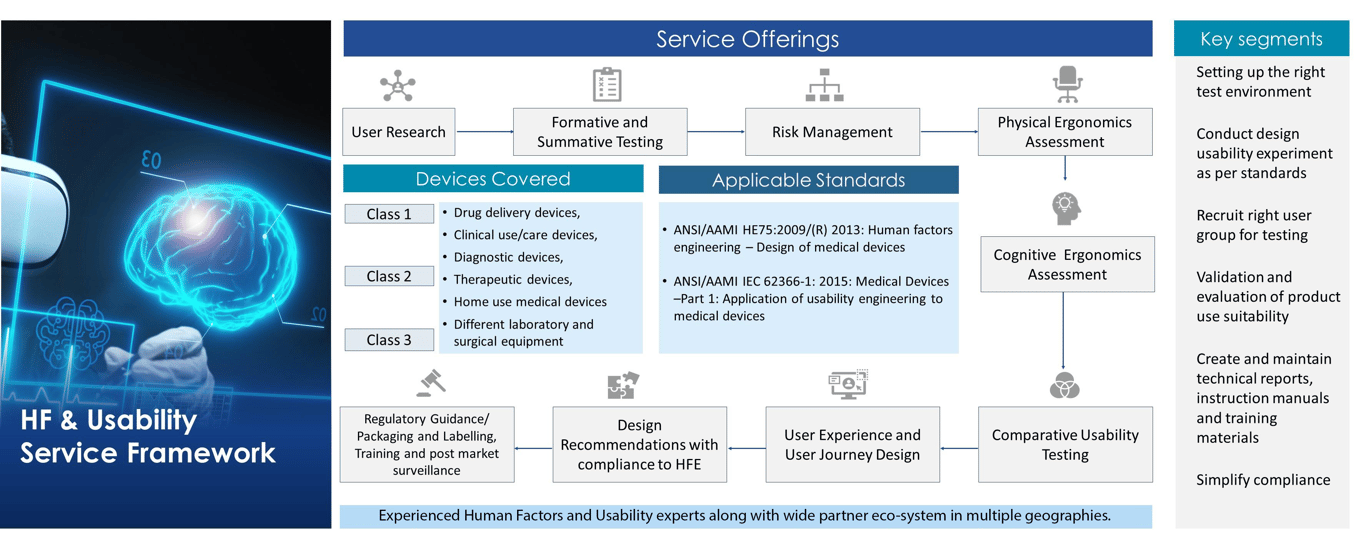
Human Factors Engineering Service Framework
Differentiators
- Technical documentation expertise catering to regulatory requirements in major and emerging markets
- In-house Medical Device Design and Engineering teams, prototyping and testing facilities
- Medium to high-risk device development experience
Benefits to Customers
- Quality devices validated according to user/ market needs
- Diverse geographical coverage and extended language purview
- Faster time-to-market with accelerated testing of medical devices
- Post-market solutions for improved designs and efficient user journey
Subscribe
To subscribe to the latest updates & newsletter