Attention
This website is best viewed in portrait mode.
-
industries
- healthcare and life sciences
- medical device regulatory compliance
-
Medical Device Biocompatibility Compliance
Medical Device Biocompatibility Compliance
Evaluate | Mitigate | Comply

Medical Device Biocompatibility Compliance
Evaluate | Mitigate | Comply
Ensuring Medical Device Safety and Reliability with Biocompatibility Evaluations

In the rapidly evolving medical device industry, biocompatibility has become a critical focus area. With increasing regulatory scrutiny and technological advancements, there is a shift towards more sophisticated and accurate methods for ensuring device safety, emphasizing the 3R principle of animal usage (Replace, Reduce, Refine).
Ensuring medical device biocompatibility presents challenges such as managing material changes, implementing robust risk management strategies, and conducting rigorous chemical profiling. Evolving regulations demand continuous refinement in evaluation strategy and a high level of expertise for more rigorous monitoring, reporting, and risk mitigation.
Tata Elxsi addresses these challenges with robust risk management, thorough chemical profiling, and the adoption of innovative in silico methods. We continuously refine our approach and evaluate alternatives to existing materials and processes, ensuring the delivery of safe, reliable, and compliant medical devices.


Here’s How We Can Help

Stringent assessment by regulatory authorities
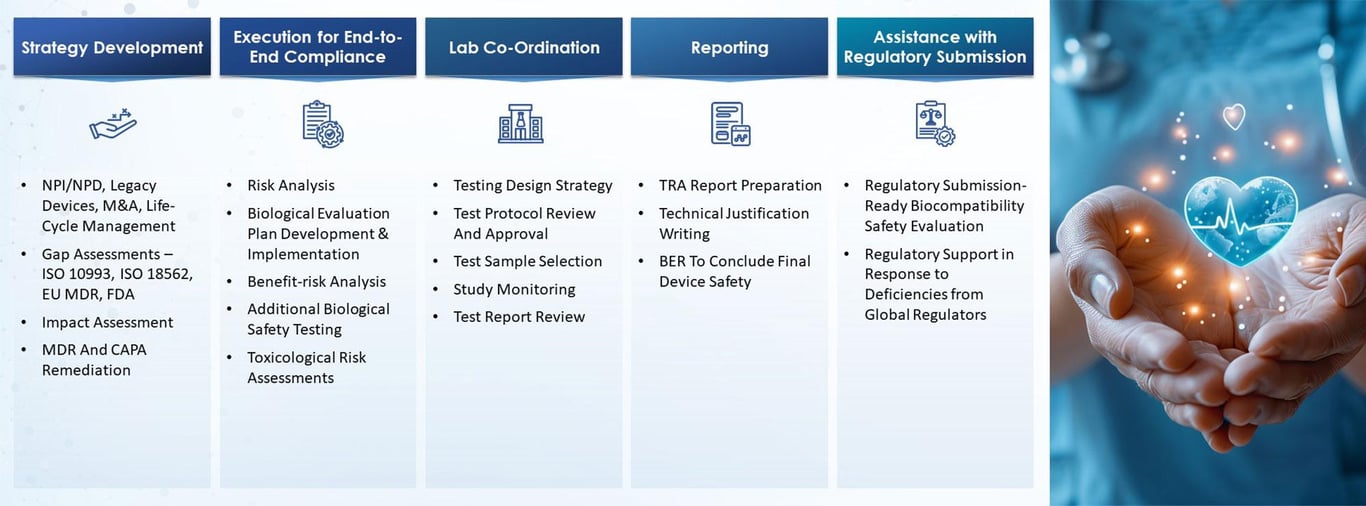
- Comprehensive biocompatibility evaluations according to the latest ISO10993, 7405, 18562 series of standard, EU MDR, US FDA and other regional biocompatibility requirements.
- Expert interpretation of complex biocompatibility data to help make critical business decisions.
- Interpretation of the new/extended requirements and frame plan of action for gap closure/remediation.
Adhering to chemicals & substances regulations for medical device raw materials & manufacturing processes
- Comprehensive chemical assessments to manage potential risks in device manufacturing.
- Toxicological risk assessment to supplement chemical analysis.
- Guidance on regulatory compliance with environmental, health & safety guidelines.
Preparing for the future of biocompatibility testing
- Theoretical methods for assessment & justification to eliminate the need of animal testing.
- Testing methods & risk assessment strategies to inform product development decisions.
- Product portfolio filtering using worst-case assessments to funnel only "must-have" products for testing.
Medical Device Biocompatibility Service Offerings
Why Tata Elxsi
- Leveraging diverse domain expertise for end-to-end biocompatibility alignment with global regulatory standards.
- Streamlining product portfolios and minimizing animal testing by up to 90% with worst-case assessments and customized solutions.
- Ensuring swift risk mitigation and timely submissions to notified bodies, expediting the compliance process.
- Managing complex supply chains with dedicated supplier coordination and vendor management.
- Agile lab coordination to save 20–25% on testing costs, improve test slot availability and reduce waiting periods.
In Focus
Subscribe
To subscribe to the latest updates & newsletter