Attention
This website is best viewed in portrait mode.
-
industries
- healthcare and life sciences
- medical device regulatory compliance
-
Medical Device Complaint Handling Services
Medical Device Complaint Handling Services
Analyze | Resolve | Optimize

Medical Device Complaint Handling Services
Analyze | Resolve | Optimize
Strategic Medical Device Complaints Management

The medical device industry is experiencing a heightened need for effective complaint handling due to increasing hazards from serious reactions and public threats. Regulatory bodies now enforce stringent timelines for submitting complaints, especially those involving serious public health threats, deaths, and significant events. Additionally, the growing awareness of evolving regulations and multiple sources of complaint reporting are shaping the industry landscape.
Manufacturers face challenges such as handling large volumes of complex complaints, ensuring timely communication, managing complaints across various channels, and maintaining regulatory compliance. Efficient data management and analysis are also critical for deriving meaningful insights from complaint data.
Tata Elxsi addresses these challenges by offering end-to-end complaint management services tailored to meet specific strategic and financial needs. Our solutions significantly reduce manual efforts and costs while providing 24/7 operational capability and regulatory expertise, ensuring comprehensive support for medical device complaint handling, processing and compliance.


Here is how we can help you?

Handling complaints of varying complexity levels and high volume
- Refining end-to-end processes with Value Stream Mapping to minimize turnaround time, enhance complaint quality, and ensure compliance.
Efficient management of complaints across multiple sources
- Developing integrated systems to handle complaints from various sources, ensuring thorough coverage and swift resolution.
- Establishing standardized processes for documenting, reporting, and tracking complaints, enhancing transparency and accountability.
Optimizing resource utilization and cost reduction
- Leveraging accelerators and technology to reduce manual efforts and costs, enhancing operational efficiency.
- Maximizing resource utilization through cross-team deployment, providing 24/7 support for complaint processing while maintaining quality and compliance.
Medical Device Complaint Service Framework
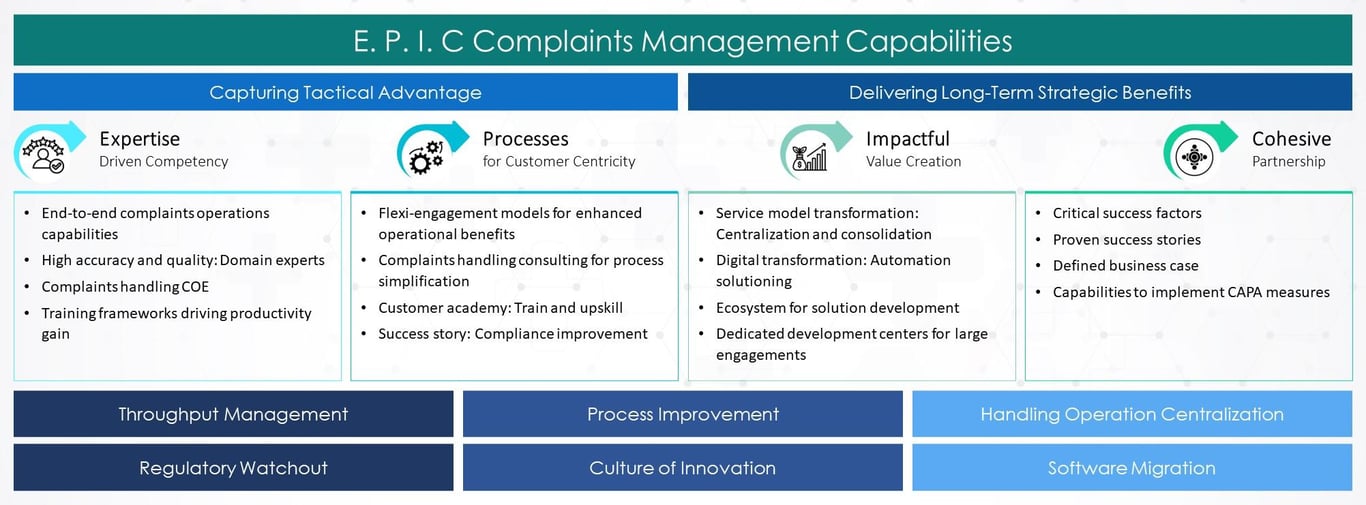
Why Tata Elxsi?
- Delivering exceptional customer value through comprehensive medical device complaint handling service and product-centric knowledge, ensuring alignment with customer needs.
- Utilizing in-house RPA-based accelerators and partnering with leading cloud-based telephony systems to significantly reduce infrastructure and hardware costs.
- Implementing Tata Elxsi’s rigorous governance and quality frameworks, with executive-level transparency and performance reporting.
- Over 200 vigilance experts and 20,000+ processed complaints, offering specialized knowledge across various therapeutic and product classes.
- Cost-efficient global delivery locations, scalable solutions, and diverse delivery models, optimizing costs by up to 40%.
Subscribe
To subscribe to the latest updates & newsletter

















