Attention
This website is best viewed in portrait mode.
-
product
- healthcare products
-
TEDREG - Medical Device Regulatory Intelligence Platform & Consulting
TEDREG - Medical Device Regulatory Intelligence Platform & Consulting
Consolidated | Validated | On-Demand

TEDREG - Medical Device Regulatory Intelligence Platform & Consulting
Consolidated | Validated | On-Demand
Medical Device Regulatory Consulting

Pharmaceuticals, biotech, and medical device manufacturers spend millions of dollars annually in compliance costs across regulated and semi-regulated markets.
With the evolving regulatory landscape and increasing competition and cost pressure, organizations are transforming their status quo to build sustainable business processes relevant to the current times. Medical device regulatory intelligence technology is invariably helping manufacturers address complex compliance requirements. Moreover, this technology is enabling companies to build an agile and proactive regulatory function capable of anticipating and responding to changes across the value chain.


Opportunities & Challenges

Embedding automated regulatory intelligence into the process value chain can help manufacturers address the dynamic regulatory developments across the globe and control OpEx on compliance.
Manual monitoring of regulations is a resource-intensive practice that adds to the company’s operational cost. Businesses are adopting automated methods to monitor and analyze multiple sources for regulatory requirements across the target regions and derive data-driven insights for portfolio strategy. Proactive regulatory intelligence allows manufacturers to disseminate information that can potentially influence future regulatory programs for an accelerated new product launch.
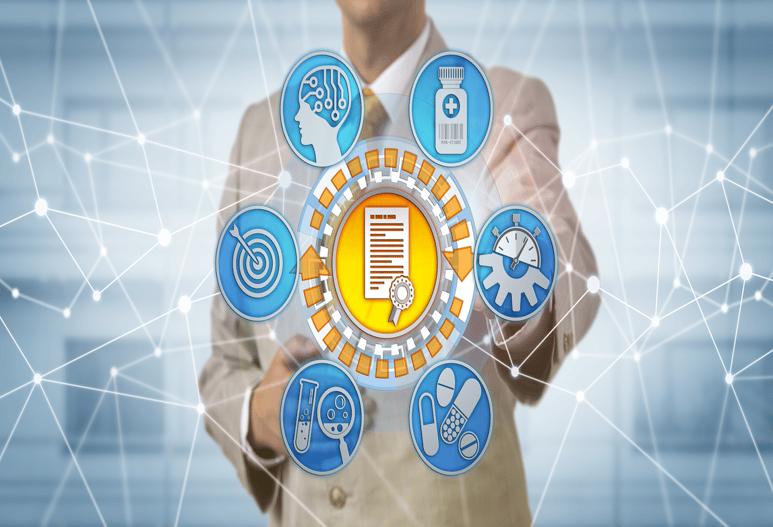
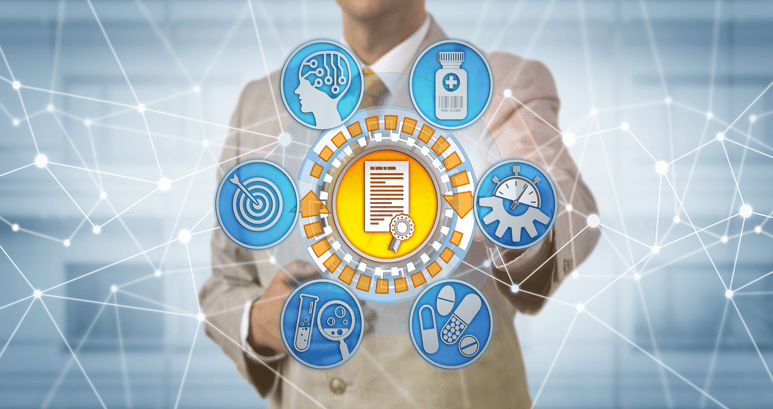
Product Framework
Features
-
Meticulous and regular monitoring of local, regional, and global regulatory information
-
Rule-based automated daily update alerts and notifications
-
Dashboards, bookmarks, and workflow management
-
Tracking specific events with search strings
-
Facilitating cross-functional collaboration by enabling discussions with peers
-
On-demand customized reports on specific topics, e.g., regulatory submissions, GVP, GCP, GMP, etc.
-
On-premise or cloud-based deployment
Differentiators
-
Validated insights – Curated intelligence validated by in-house/ external regulatory experts
-
Flexibility for “on-demand” or “periodic & structured” reports
-
On-demand consulting with medical device regulatory experts
-
Commercial models configured for subscription-based, demand-based, and functional service outsourcing
Benefits to the Customer
-
Auto-crawling reduces manual search efforts
-
Assimilation of data in one single repository reduces information distribution time
-
Real-time information availability ensure speed of process that impact time-to-market by more than 20%
-
Reduced localization expense can save OpEX cost
Subscribe
To subscribe to the latest updates & newsletter