Attention
This website is best viewed in portrait mode.
-
services
- test and validate
-
Verification & Validation
Verification & Validation
Design | Assess | Certify
Verification & Validation
Design | Assess | Certify
Trending
Amidst the relentless competition in the MedTech industry, manufacturers are developing optimized test strategies, leveraging automation and DevOps to accelerate time-to-market.
Comprehensive and rigorous Verification & Validation plans are being implemented to address the ever-changing regulatory requirements set forth by the authorities. Furthermore, to enable better clinical outcomes, manufacturers are developing holistic solutions by integrating digital and novel technologies into their products. Moreover, enabling a seamless user experience across the value chain demands an additional emphasis on tests such as compatibility testing, security testing, etc.

Opportunities & Challenges

In a highly regulated and competitive industry, the key challenge for the manufacturers is to ensure product quality, reliability, and patient safety throughout the medical device lifecycle.
While an early launch of a medical device in the market could lead to significant market share and maximum ROI, manufacturers with ineffective testing strategies go through prolonged testing cycles. This may also potentially lead to product recalls high cost of maintenance and dwindling customer confidence. To achieve product commercialization goals, companies are finding ways to accelerate product development through automation-based optimized V&V programs.
Service Framework
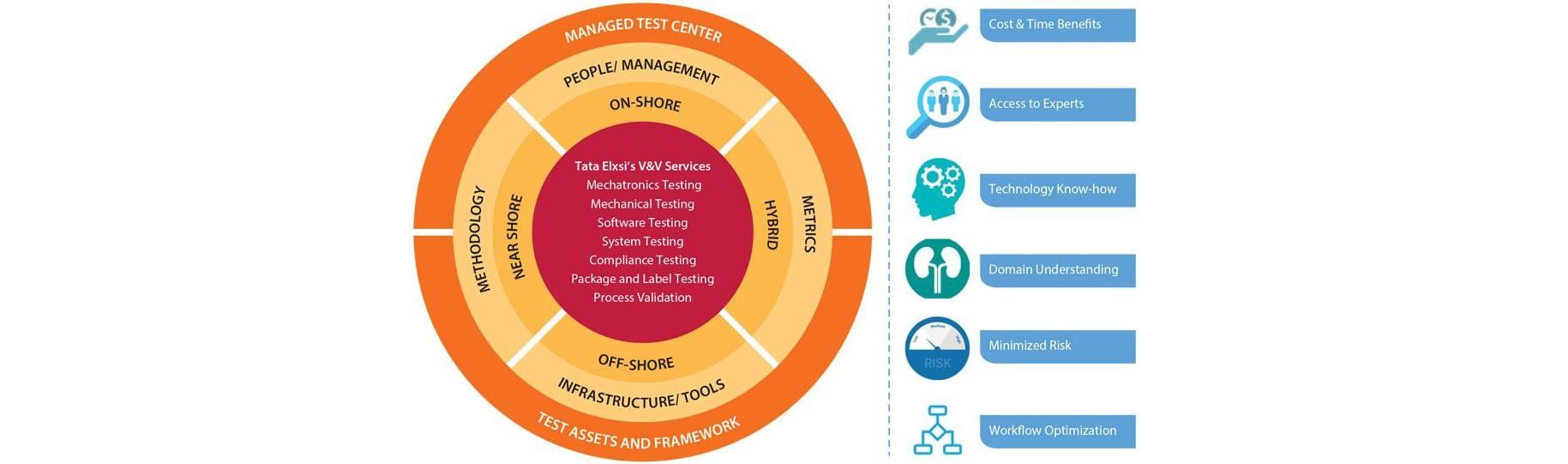
Verification & Validation with Tata Elxsi
-
Dedicated V&V center of excellence
-
Mature defect management process and KPI-based governance
-
Automation experience on multiple COTS & homegrown tools
-
An ecosystem of internationally accredited testing labs
Differentiators
-
15+ years of safety-critical device V&V experience
-
ISO 13485 certified facilities with QMS aligned to meet ISO 14971, IEC 62366, IEC 62304, 21 CFR part 820, and EU regulations
-
Team of industry-trained biomedical and systems engineers with medium to high-risk device testing experience
-
Technical documentation expertise catering to regulatory requirements in major and emerging markets
Benefits to the Customer
-
High-quality devices to ensure patient safety
-
Patient data security across the healthcare value chain
-
Quick turnaround time for device maintenance and minimum downtime
-
Faster time-to-market with accelerated testing of devices
-
Affordable and quality devices validated for user/ market needs
Subscribe
To subscribe to the latest updates & newsletter











